Infrared nanospectroscopy in liquid
Introduction
Infrared nanospectroscopy (nanoIR) has gained widespread attention in the academic community since 2013. However, early nanoIR systems faced challenges in analyzing samples in aqueous environments due to the strong absorption of infrared light and damping of the AFM probe's oscillation. Characterizing samples in liquid environments, particularly on electrochemical interfaces, has been a long-standing challenge.
With support from national scientific research equipment over the past 5 years, our team has developed a sophisticated nanoIR system and thoroughly studied its fundamental aspects, including physical principles, measurement technology, environmental control, and sample preparation methods. Our efforts have resulted in the development of three generations of nanoIR systems, which have addressed and solved a range of technical challenges.
The first generation of the system focused on establishing the technical foundation and addressing basic technical issues, such as software, hardware, data acquisition, and online algorithms. The second generation made significant advancements by demonstrating nanoIR experiments in aqueous environments with a bottom excitation module. The current third generation prioritizes innovation, and through the use of a customized electrochemical cell, it allows for the first-ever detection of in-situ nanoIR signals of electrochemical reactions on solid/liquid interfaces. The third-generation system also has the ability to provide multimode imaging at the nanoscale, including correlations of morphology, mechanics, and electrical information. We are working towards further improving and expanding the capabilities of nanoIR.
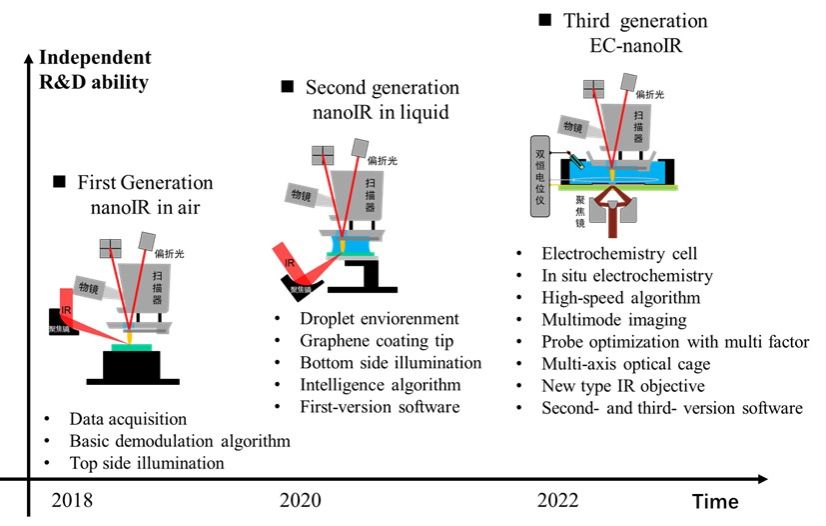
Figure 1. Schematic diagram of self-developed 3 generations of nanoIR instruments and the corresponding technological breakthroughs phased independently.
The third-generation nanoIR system, featuring a customized spectroelectrochemical cell as its core component. The cell consists of a BaF2 substrate coated with a 10-nm gold film as the working electrode and is illuminated directly from the bottom via a reflective objective. The spectroelectrochemical cell is mounted on a three-axis nanopositioning stage and integrated into a multi-axis cage system for stability and compatibility..
To facilitate the system, a comprehensive set of measurement and control software has been developed, encompassing mid-IR laser control, AFM operation, electrochemical functions, and data processing modules. The software features an embedded algorithm to optimize IR pulse excitation efficiency and transmit the nanoIR signal to the AFM controller for simultaneous display with correlated morphology. Additionally, an electrochemical control module has been added to allow for electrochemical nano-infrared spectroscopy and imaging measurements. The nanopositioning stage also enables sample scanning, which can be combined with an intelligent algorithm for high-speed nanoscale infrared hyperspectral imaging. Utilizing the third-generation electrochemical nanoIR system, we have achieved a significant breakthrough by acquiring in-situ infrared spectra and imaging of the electrochemical interface for the first time.
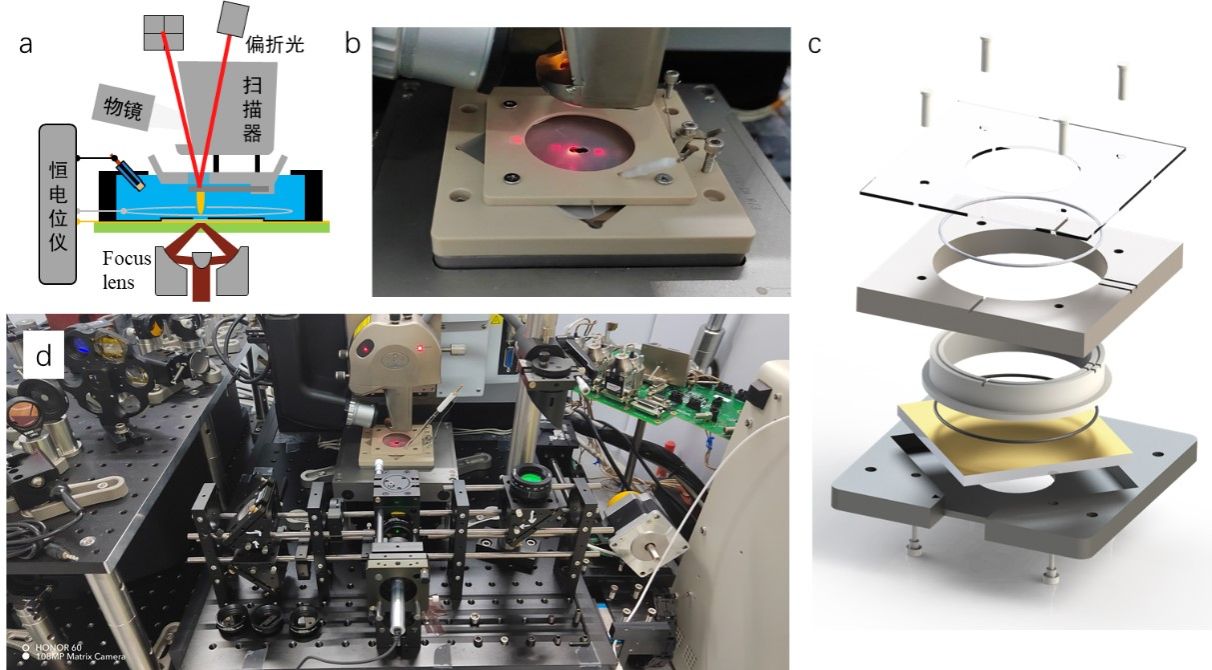
Figure 2. (a) Design schematic diagram of the latest third-generation instrument. (b) Photograph of spectroelectrochemical cell loaded in the system. (c) Structural design drawing of the spectroelectrochemical cell. (d) Physical photo of the third generation of instruments and devices, including multi-axis cage structure and spectroelectrochemical cells
Performance
The polymethyl methacrylate (PMMA), polycarbonate (PC), polystyrene (PS), and block copolymers of PMMA and PS films are suitable benchmark samples for nanoIR characterization. AFM probe scans on the surface of PMMA film exhibit distinct differences in air versus aqueous environments. In air, the probe's free vibration amplitude persists, while in solution, it rapidly decreases. The "jump to contact" point is more pronounced in air, but less so in solution.
The signal-to-noise ratio of nanoIR spectra remains good in both air and solution conditions, though the baseline of the spectrum is higher in solution. By imaging the distribution of C=O bonds at a fixed excitation wavelength of 1730 cm-1, the team demonstrated the stable and high performance of nanoIR microscopy in aqueous environments. The correlated nanometer-scale imaging of morphology and chemical information showed heterogeneous distribution of the C=O bonds, verifying the effectiveness of the system.
To demonstrate the sensitivity of the system, we prepared ultra-thin samples on an ultra-thin gold film substrate using the Langmuir-Blodgett (LB) method. We found a strong correlation between the AFM topography imaging and the nanoIR imaging at 1630 cm^-1. The comparison of the topography profile and nanoIR intensity indicated that the system achieved a spatial resolution of 3.16 nm perpendicular to the edge of the LB film, surpassing the previously reported resolution of 6 nm. Moreover, we imaged DNA double helix chains at 1280 cm^-1 and 1700 cm^-1 and observed helix-like structures at the edge of the DNA strands, demonstrating the super sensitivity and spatial resolution of the third-generation system on a 1.2 nm thick sample.
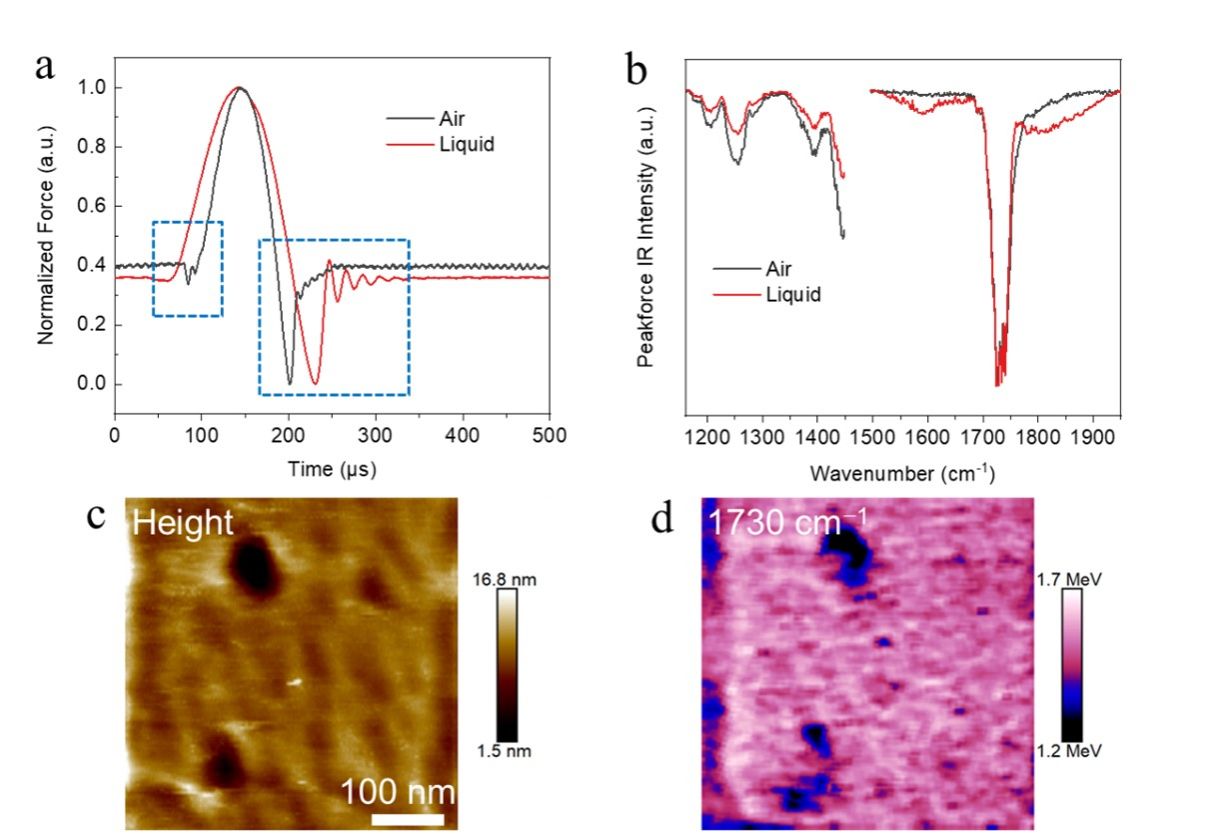
Figure 3. NanoIR measurement of the polymer film surface of a standard sample in the aqueous environment (a) The force curves of the tip on the surface of the polymer film in aqueous solution and air respectively. The box shows the obvious difference between the two curves. (b) Comparison of nanoIR spectra of PMMA film in air and water solution. (c) Surface topography of PMMA film. (d) C=O bond imaging on the surface of PMMA film.
To testify the sensitivity of the instrument, we prepare few-layer ultra-thin samples on the surface of ultra-thin gold film substrate via the LB (Langmuir-Blodgett, LB) method. The AFM topography imaging is highly relevant to the nanoIR imaging at 1630 cm-1. Comparing the topography profile and nanoIR intensity, the results show that the spatial resolution of the instrument reaches 3.16 nm perpendicular to the edge of the LB film, which is better than the reported 6 nm spatial resolution. Further, for confirmation, DNA double helix chains are imaged at 1280 cm-1 and 1700 cm-1 respectively, in which helix-like structures can be observed at the edge of the DNA strand. The experimental result shows that the third-generation system is of super sensitivity and spatial resolution on the 1.2-nm thick sample.
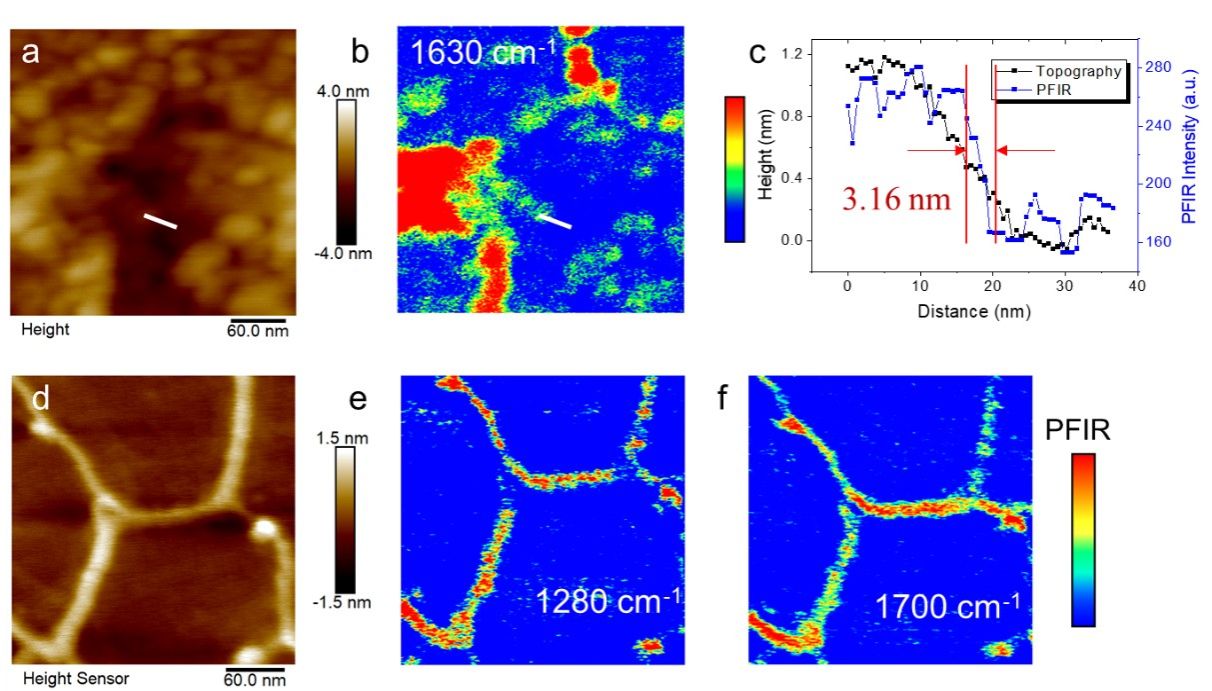
Figure 4. Ultra-sensitive and ultra-high spatial resolution nano-infrared imaging measurement of ultra-thin sample film.(a) AFM imaging of ultrathin samples prepared by LB film technique. (b) the correlated nano-infrared imaging excited at 1630 cm-1. (c) the contour of white line area in morphology and nano-infrared imaging, and the correlation between nano-infrared intensity and appearance. (d) morphological imaging of DNA strands. (e) nano-infrared imaging excited at 1280 cm-1. (f) nano-infrared imaging excited at 1700 cm-1.
In-situ Nano-IR Spectroscopic Observation of Electrochemical Processes
We have demonstrated the ability of our third-generation nanoIR system to measure in-situ electrochemical reactions by performing an aniline electropolymerization. The infrared beam passed through a BaF2 substrate to reach the probe-sample nanogap. The reaction was performed in an electrochemical cell containing 0.1 M H2SO4 and 30 mM aniline. The reaction area at the electrode interface underwent a color change from light yellow, reflected by the 10 nm gold film, to dark green polyaniline film after polymerization. The process was monitored by cyclic voltammetry and showed a shift and increase in the first oxidation and reduction peaks of aniline electropolymerization over multiple cycles, indicating both polymerization and electrochemical proton doping. The infrared absorption spectra of the film after the reaction showed three main peaks corresponding to the benzene ring structure and quinone structure of the film. When the IR wavenumber was fixed at 1500 cm-1, the vibration signal of the benzene ring skeleton at the surface of the gold electrode became stronger during the reaction and showed heterogeneous distribution associated with the morphology pattern.
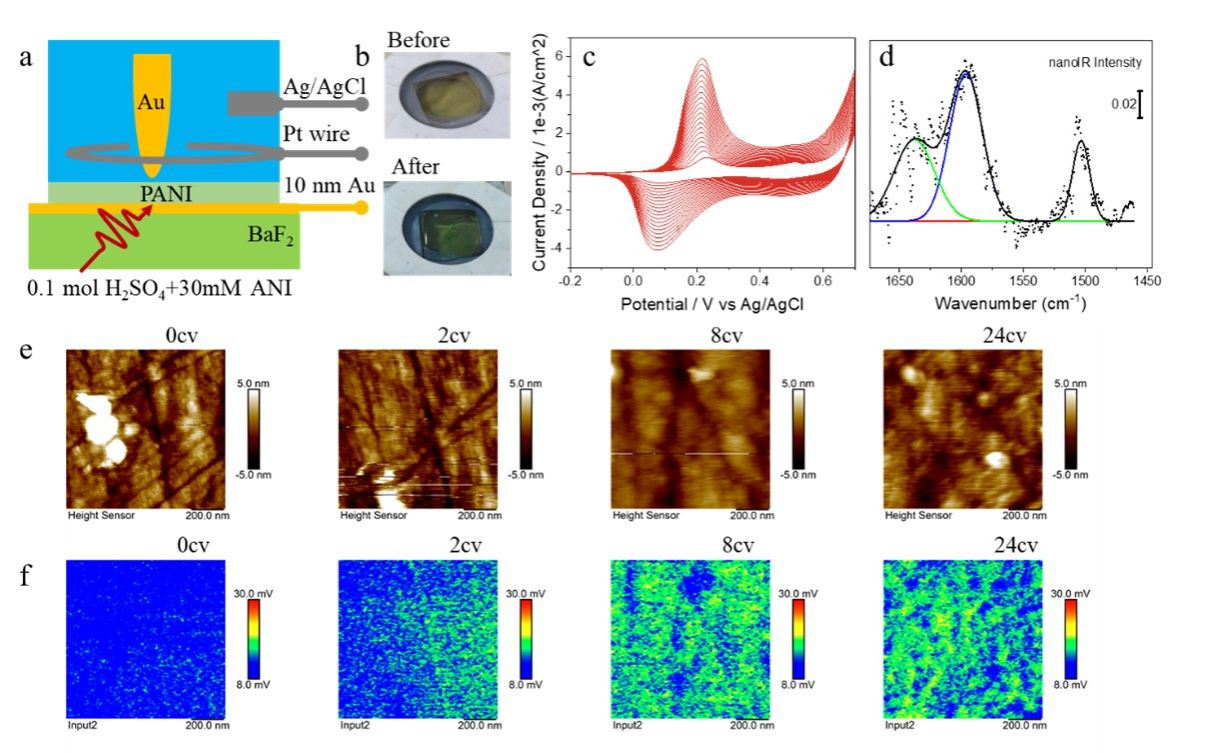
Figure 5. In situ nanoIR observation of aniline electropolymerization process. (a) Schematic diagram of experimental device structure. (b) Color comparison of electrochemical cell electrode reaction region before and after polymerization. (c) The voltammetry curve of aniline electropolymerization at -0.2 V-0.7 V for 40 cycles. (d) nanoIR spectrum of the polyaniline film on the electrode surface, in which the scatter map is the measured data, and the black curve is the fitting spectrum. (e) Morphology evolution of electrode interface in electrochemical process. (f) nanoIR imaging at 1500 cm-1 associated with morphology evolution.
