SpectroElectroChemistry
From the preparation of SERS-active substrates, to methodology and theory, our group aims to expand the breadth and deepen the understanding of electrochemical SERS (EC-SERS) in surface science and material science.
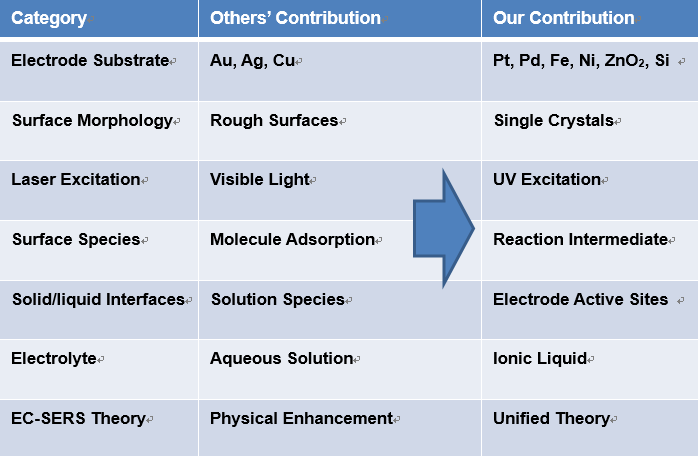
EC-SERS on chemisorptions
For the EC-SERS characterization, our group has invented and developed diverse methods to prepare the electrochemically roughened or nanoparticles assembled film electrodes. Especially, the contributions to expand EC-SERS on transition metal (VIII B) surface of which the original SERS activity is rather limited. With the excellent SERS-active substrates, we have obtained high-quality electrochemical Raman signal of pyridine adsorbed on the coinage metal and transition metal surfaces. Concurrently, to improve our fundamental understanding of the electrode/electrolyte interface, we have successfully observed the first SERS (also the first Raman) signal of surface water on Pt-group metals.
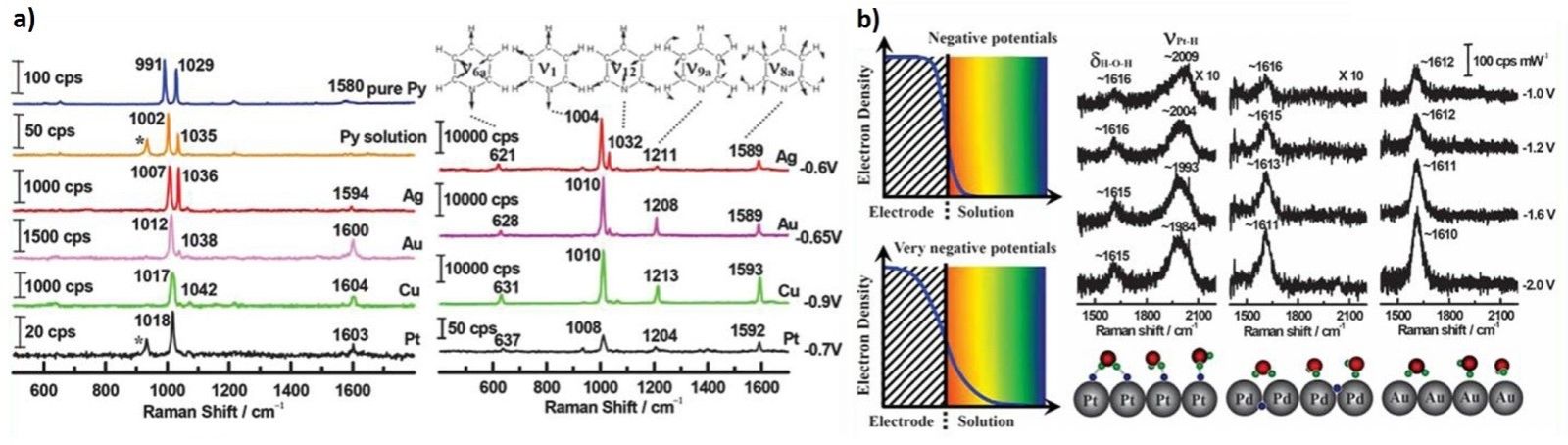
Figure 1. a) SERS spectra of pyridine adsorbed on roughened Ag, Au, Cu and Pt electrodes at open circuit potential (left) and the peak potential (vs. SCE) of the ring breathing mode (right); b) SERS spectra of water adsorbed on Pt, Pd and Au at negative potentials in 0.1 M KClO4 (right top). The suggested models (right bottom) for the adsorbed water on different electrodes and the influence of potential on metal conduction electron are shown on the left. The suggested models (right bottom) for the adsorbed water on different electrodes and the influence of potential on metal conduction electron are presented.
EC-SERS on reaction processes and electrode kinetics
When combined with electrochemical techniques and theoretical calculation method, in-situ EC-Raman will shed the light on the in-depth understanding of reaction mechanisms in catalysis, electrochemistry, organic chemistry, etc. Take the reduction of benzyl chloride on silver electrode for instance, we carried out an in situ electrochemical surface-enhanced Raman spectroscopic study to characterize various surface species in different electrode potential regions. Corresponding DFT calculation reveals that the benzyl radical and its anionic derivate bonded on a silver electrode are the key intermediates, implying that the pathway could drastically differ from the outer sphere concerted electron reduction at inert electrodes.
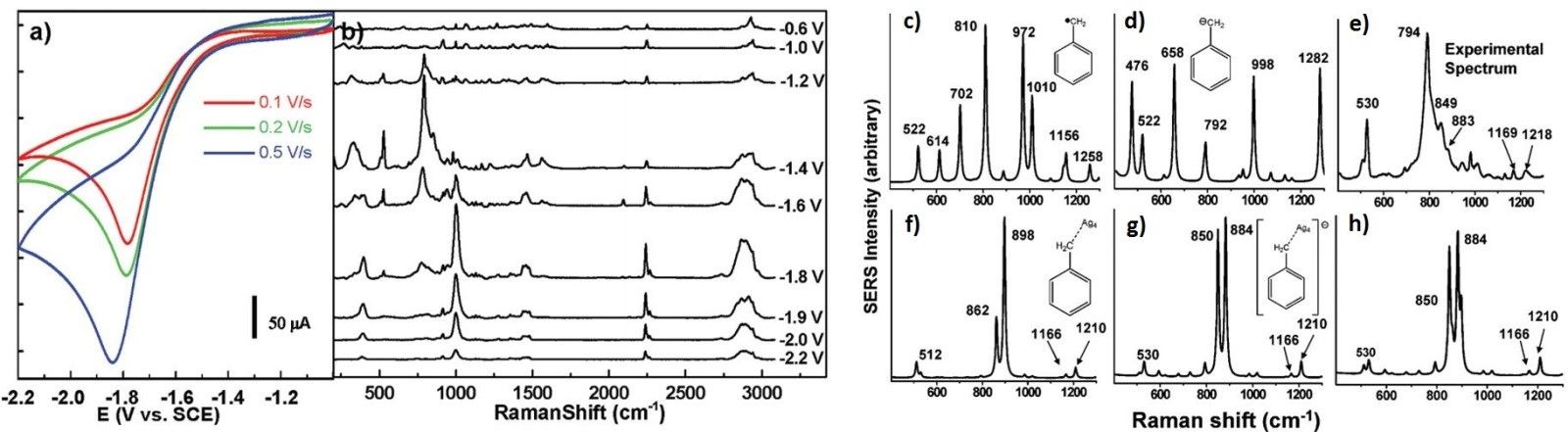
Figure 2. a) CV of 5 mM PhCH2Cl in 0.1 M TEAP + CH3CN at a Ag electrode with different scan rates; b) Potential dependent SERS spectra of PhCH2Cl on a Ag electrode; DFT calculated Raman spectra of the possible solvated reaction intermediates: c) free benzyl radical, d) free benzyl anion, f) benzyl radical-Ag4 adduct, g) benzyl anion-Ag4 adduct. These are compared with e) the experimental SERS spectrum at -1.4 V vs SCE and h) a 1:5 superposition of the predicted spectra in f and g.
EC-SHINERS for in-situ monitoring reactions on single crystal surfaces
Single crystal surfaces are commonly preferred and used in surface science, because of their well-defined surface state and optic field. However, SERS is seriously limited to roughened or nanostructured surfaces. The electrooxidation processes play the crucial role in electrocatalysis investigations. Herein, electrochemical shell-isolated nanoparticle-enhanced Raman spectroscopy (EC-SHINERS) is utilized to in situ monitor the electrooxidation processes at Au(hkl) single crystal electrode surfaces. The experimental results are well correlated with our periodic density functional theory calculations and corroborate the long-standing speculation based on theoretical calculations in previous electrochemical studies. The presented in situ electrochemical SHINERS technique offers a unique way for a real-time investigation of an electrocatalytic reaction pathway at various well-defined noble metal surfaces.
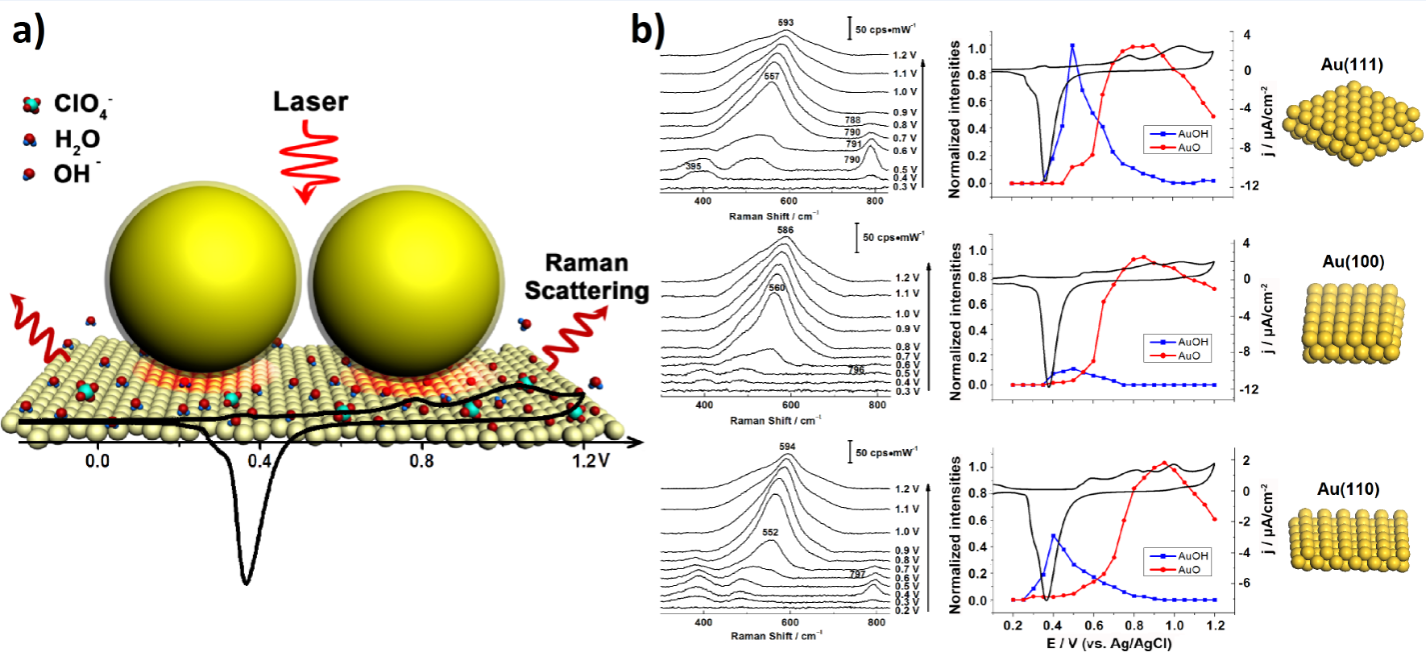
Figure 3. a) Schematic diagram of EC-SHINERS at single crystal surface; b) The EC-SHINERS spectra of the electrooxidation processes of Au(111), Au(100), and Au(110) electrodes in 0.1 M NaClO4 (from top to bottom). The corresponding CVs are presented as well.
References
1. Tian, Z. Q. & Ren, B. Adsorption and reaction at electrochemical interfaces as probed by surface-enhanced Raman spectroscopy. Annu. Rev. Phys. Chem 55, 197–229 (2004).
2. Wu, D., Li, J., Ren, B. & Tian, Z. Electrochemical surface-enhanced Raman spectroscopy of nanostructures. Chem. Soc. Rev. 37, 1025-1041 (2008).
3. Wang, A. et al. In Situ Identification of Intermediates of Benzyl Chloride Reduction at a Silver Electrode by SERS Coupled with DFT Calculations. J. Am. Chem. Soc. 132, 9534–9536 (2010).
4. Li, J. et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 464, 392-395 (2010).
5. Li, C. Y. et al. In Situ Monitoring of Electrooxidation Processes at Gold Single Crystal Surfaces Using Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy. J. Am. Chem. Soc. 137, 7648-7651 (2015).
